What is nanotechnology?
There are a number of departments and agencies within the U.S. federal government that define ‘nanotechnology.’ The National Nanotechnology Initiative (NNI) is a U.S. federal government R&D initiative that defines nanotechnology as “the understanding of and the control [or manipulation] of matter at the nanoscale.” Nanoscale is defined as particle sizes between 1 and 100 nanometers (nm) (1 nm = 1 x 10-9 m).
Nanoscale materials may exhibit different physical and chemical properties compared with the same substances at normal scale. However, nano is not completely understood today; it involves a variety of fields such as physics, chemistry, biology, material science and engineering.
Is nanotechnology new? What are some examples of nanotechnology?
Nanoscale particles are not new in nature or science. Things such as hemoglobin, smoke from a fire, volcanic ash, and sea spray are all forms of naturally occurring nanoscale material.
Are there foods on the market containing nanotechnology?
Yes. Broadly speaking there are two categories of foods that contain nano-sized materials. One category is foods that have always contained ingredients that fit the size definition of nanoscale. An example is mayonnaise, an oil-in-water emulsion, where oil is dispersed in water so that the tiny particles don’t separate the way oil and water typically does. Another example is the network formed by gelatin at the nano-scale.
The second category of products are the ones that contain new types of ingredients that have been produced at the nanoscale. These are the ones that are getting more consumer attention. Applications include adding vitamins to products in a way that makes them easier for the body to use. In addition to improving nutrition, improving taste, flavor and texture are other ways in which nano-sized food ingredients can play a positive role.
Are foods that are produced using nanotechnology safe?
The considerations of and questions around nanotechnology and food safety are: What are the human and environmental impacts? Right now, there are many unanswered questions. It is the responsibility of food manufacturers utilizing nanotechnology to make sure that the products they produce are safe, and the science around the safety of ingesting new nanoparticles is still evolving.
As nanotechnology gains hold it is important to focus on how nanoscale materials are evaluated. Currently, we are in our infancy with respect to figuring out how to evaluate new nanomaterials for safety when they will be used in foods.
The World Health Organization (WHO) has issued a technical paper on nanotechnologies in which it states that the available information for this topic is limited. The WHO identifies that most research has June 2015 focused on occupational exposure to nanoscale materials, as opposed to the ingestion of foods containing nanoparticles. They identify the need for assessments in several areas in order to understand ‘nanotoxicology’:
- Composition
- Characterization of the chemical properties
- Biopersistence
- Digestibility
- Impurities
This information will be critical in understanding whether and in what form the nanoscale material may be transported through the body (animal or human) and potentially enter into a cell and elicit a toxic response.
In 2013, the European Food Safety Authority (EFSA) reported their findings that, based on studies performed by Member States, updated protocol for in vitro and in vivo test methods to evaluate nanoscale material would be needed. Also, a 2011 review in the Journal of Food Science concluded that “there is a need for additional toxicology studies of sufficient quality and duration on different nanomaterials to further understand the characteristics of nanomaterials that affect the safety of oral exposure resulting from use in various food applications.”
How can I tell if foods are produced using nanotechnology?
Currently, there is no US requirement that foods be labeled regarding the use of nanotechnology or nanomaterials. Similarly in the European Union, there is no current requirement to indicate that ingredients are derived using nanotechnology, although this has been the subject of much debate, including parliamentary votes.
As a result, it can be difficult for consumers to know if a food has been produced using nanotechnology. A consumer should contact the food manufacturer to get more information.
The Woodrow Wilson Center’s Consumer Products Inventory (http://www.nanotechproject.org/cpi/) contains a searchable database of various consumer products that include materials derived from nanotechnology.
Is nanotechnology in foods regulated by the government? Does the government need to approve foods made using nanotechnology?
The FDA Fact Sheet on nano states that “Understanding nanotechnology remains a top FDA priority. FDA is monitoring the evolving science and has a robust research agenda to help assess the safety and effectiveness of products using nanotechnology.” In April 2012, FDA issued draft guidance describing factors that manufacturers should consider when determining whether a significant change in manufacturing process – including that of nanotechnology – for a food substance already in the market affects the identity, safety of use, or regulatory status of use of the food substance. The guidance states, “FDA considers food manufacturing processes that involve nanotechnology in the same manner as any other food manufacturing technology.” Thus, there is no specific testing required for its use. Rather, if there is no significant change in manufacturing process with the use of nanotechnology, the agency sees it as not affecting food safety.
Specifically focusing on nanotechnology, FDA states that if the food substance were manufactured “for the purpose of creating very small particle sizes with new functional properties,” it would likely not be covered by an existing Generally Recognized As Safe (GRAS) determination because the determination requires both technical evidence of safety and a basis to conclude that this technical evidence of safety is generally known and accepted. Additionally, FDA states, “at present, for nanotechnology applications in food substances, there are questions related to the technical evidence of safety as well as the general recognition of that safety.” Thus, FDA’s basis for requiring a food additive petition be filed relates to whether or not the nanomaterials confer new functional properties, rather than just viewing the presence of such materials as grounds for requiring a petition.
There have been localized efforts aimed at addressing nano-technology. While not food-specific, in Berkeley, California, there has been a requirement since 2006 that laboratories working with any nanomaterials report the use to the city (http://step.berkeley.edu/White_Paper/Barandiaran.pdf; http://digitalcommons.pace.edu/cgi/viewcontent.cgi?article=1721&context=pelr).
An EU regulation around food additives states that if the particle size differs (such as by the use of nanotechnology, listed as an example), then it is considered a “different” additive and needs to be considered separately.
In Canada, the use of nanotechnology is not permitted for foods that are labeled “organic.”
Do consumers embrace or oppose nanotechnology?
At this time, relatively few consumers seem to be focused on nanotechnology. However, as the use of nanomaterials in foods grows, it can be expected that consumer awareness will grow, prompting questions around which foods contain nanoparticles and their safety.
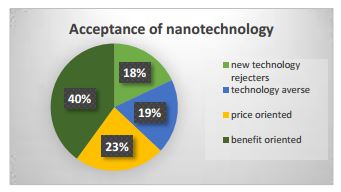
A study of consumer attitudes found that about a quarter of consumers are motivated by price rather than the production techniques, a fraction will oppose any new technology, and the remainder are open to the technology if the benefits are clear—although some need more information to make those decisions than others (http://onlinelibrary.wiley.com/doi/10.1111/1477-9552.12090/abstract).The chart below highlights the different populations.
Bottom line
The science and politics of nanotechnology continues to evolve and consumers may begin asking questions around foods containing nanomaterials, similar to the types of questions and concerns expressed around Genetically Modified Organisms (GMOs).
Whether ingredients occur naturally or were engineered, it is difficult to identify which food products do and don’t contain ingredients in the nano-scale. Retailers should be aware of the Consumer Product Inventory database as a resource to their customers http://www.nanotechproject.org/cpi/
Additional Information
Andrea Bieberstein, Jutta Roosen, Stéphan Marette, Sandrine Blanchemanche and Frederic Vandermoere. “Consumer Choices for nano-food and nano-packaging in France and Germany.” European Review of Agricultural Economics. 2013, 4 (1). Accessed March 6, 2014. http://erae.oxfordjournals.org/content/40/1/73.short
BA Maqnuson, Jonaitis TS and Card JW. A Brief Review of the Occurrence, use, and Safety of Food – Related Nanomaterials. J Food Science. 2011, 76(6):R126-33.
European Food Safety Authority. Technical Report: Annual report of the EFSA Scientific Network of Risk Assessment of Nanotechnologies in Food and Feed for 2013. Accessed Dec 5, 2014. http://www.efsa.europa.eu/en/supporting/doc/531e.pdf
FDA nanotech fact sheet: http://www.fda.gov/ScienceResearch/SpecialTopics/Nanotechnology/ucm402230.htm
The Organic and Non-GMO report, “Canada bans nanotechnology in organics.” Accessed Dec 5, 2014 http://www.non-gmoreport.com/articles/may10/canada_bans_nanotechnology_organics.php
World Health Organization. ‘State of the art on the initiatives and activities relevant to risk assessment and risk management of nanotechnologies in the food and agriculture sectors.’ (Draft Public Review). Accessed Dec 5, 2014. http://www.fao.org/docrep/018/i3281e/i3281e.pdf
- Food Safety & Security
 Industry Topics address your specific area of expertise with resources, reports, events and more.
Industry Topics address your specific area of expertise with resources, reports, events and more.
 Our Research covers consumer behavior and retail operation benchmarks so you can make informed business decisions.
Our Research covers consumer behavior and retail operation benchmarks so you can make informed business decisions.
 Events and Education including online and in-person help you advance your food retail career.
Events and Education including online and in-person help you advance your food retail career.
 Food Safety training, resources and guidance that help you create a company food safety culture.
Food Safety training, resources and guidance that help you create a company food safety culture.
 Government Affairs work — federal and state — on the latest food industry policy, regulatory and legislative issues.
Government Affairs work — federal and state — on the latest food industry policy, regulatory and legislative issues.
 Get Involved. From industry awards to newsletters and committees, these resources help you take advantage of your membership.
Get Involved. From industry awards to newsletters and committees, these resources help you take advantage of your membership.
 Best practices, guidance documents, infographics, signage and more for the food industry on the COVID-19 pandemic.
Best practices, guidance documents, infographics, signage and more for the food industry on the COVID-19 pandemic.
